FDA is Recommending Pausing the Use of Johnson and Johnson Vaccine
CDC & FDA Recommends Pausing Johnson & Johnson Vaccine
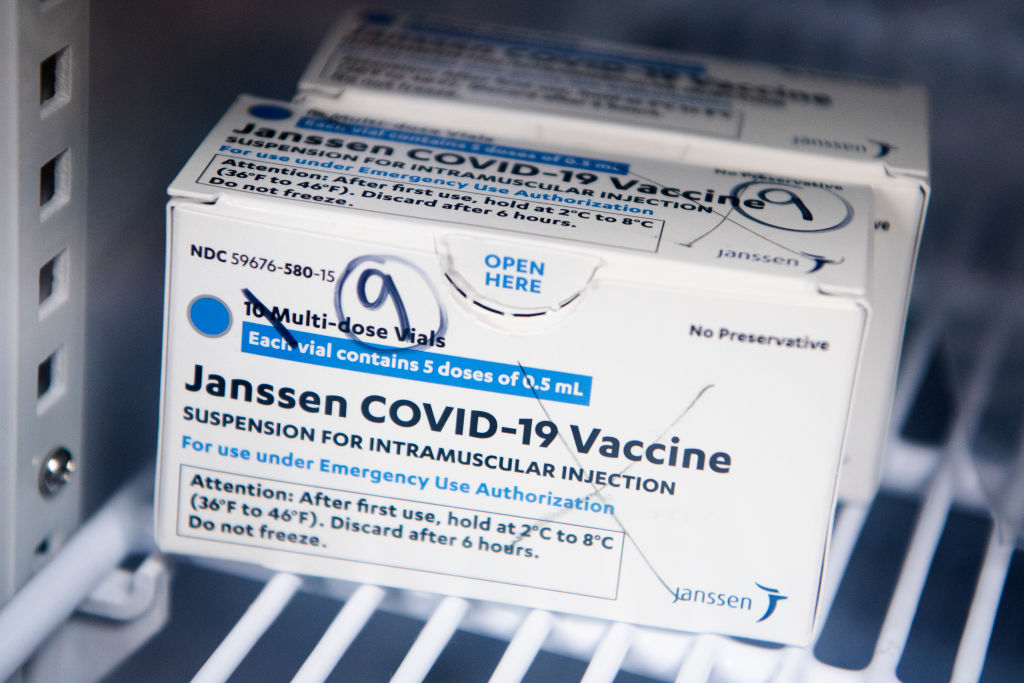
Source: Tom Williams / Getty
The FDA has announced the distribution of the Johnson & Johnson COVID-19 vaccine will be paused after six women in the U.S. had rare blog-clotting after receiving the vaccine. The women ranged in ages from 18 to 48, and while six people may not seem like a lot precautions are being taken swiftly. There have been approximately 6.8 million shots given with the majority of people not having issues.
The pausing of the Johnson & Johnson vaccine does not affect the other two vaccines from Pfizer and Moderna. But if you did receive the Johnson & Johnson shot and are having to severe stomach pain, leg cramps, or severe headaches you should call your healthcare provider right away. If you do not know what shot you received, check your CDC vaccination card where it is noted what brand shot you received. If you have an appointment scheduled to receive the Johnson & Johnson COVID-19 vaccine, it is advised to call your provider for further directions.
Get Breaking News & Exclusive Contest in Your Inbox:
The Latest:
- 2026 RodeoHouston Tickets Now On Sale
- Meet Zinzi Coogler – The Woman Behind ‘Sinners’ Everyone’s Talking About
- Who Is Lady London?: The Rapper And Scholar Redefining Hip-Hop
- LeBron James Distances Himself From Trade Hot Take After Austin Reaves’ Rep Pulls Up On Rich Paul
- Olandria Carthen Stuns At ‘Bridgerton’ Ball
- Mike Tomlin Steps Down As Pittsburgh Steelers Head Coach After 19 Historic Seasons
- Dr. Ian Smith Explains Why the Last 15 Pounds Are the Hardest to Lose
- Historic Milestone: TSU With Another First
- H-Town News: Civil Rights Pioneer Claudette Colvin Dies
- LeBron James Quotes HOV
CDC & FDA Recommends Pausing Johnson & Johnson Vaccine was originally published on mycolumbusmagic.com



